Abstract
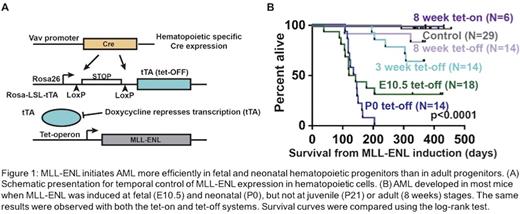
Translocations involving the MLL1/KMT2A gene are observed in both acute lymphoblastic leukemia (ALL) and acute myeloid leukemia (AML), but the frequency at which these mutations occurs is different at different stages of life. For example, MLL rearrangements are extremely common in infant leukemia (~70% of cases), but they are much less common in adolescent or adult AML (~5% of de novo adult AML). While the function of MLL fusion proteins has been extensively studied, it remains unclear why MLL-rearrangements are so common in infant leukemias as compared to adolescent or adult leukemias. One possibility is that fetal hematopoietic progenitors are more readily transformed by MLL fusion proteins than adult progenitors. Recent work in our lab has shown that a mutation commonly associated with adult AML, the FLT3-Internal Tandem Duplication, selectively activates leukemogenic transcriptional programs in adult as compared to fetal/neonatal progenitors (eLife 5:e18882). We have developed a system to test whether an analogous effect holds for MLL translocations - i.e. do they preferentially initiate leukemogenesis in fetal progenitors. We made use of a previously described tet-O doxycycline inducible mouse allele (Cell Reports 9:1246) to both spatially and temporally control expression of MLL-ENL, a common MLL fusion product (Fig. 1A). We found that MLL-ENL induction at fetal (E10.5) or neonatal (P0) stages caused AML to develop in most mice, but MLL-ENL induction at juvenile (P21) or adult (8 weeks) stages did not (Fig. 1B). Remarkably, AML developed with the greatest penetrance (100%) when MLL-ENL was induced at P0 as compared to both earlier and later developmental stages. In neonates, MLL-ENL profoundly depleted hematopoietic stem cells (HSCs) and, to a lesser extent, lineage-restricted hematopoietic progenitors (HPCs), and it caused expansion of myeloid progenitor populations. These effects began to dissipate by 10 weeks after birth. Changes in HSC and HPC frequency were not at all evident when MLL-ENL was induced at an adult stage, but by a year after MLL-ENL induction, the surviving mice had a significantly expanded HSC pool. MLL-ENL induced widespread changes in gene expression in neonatal HPCs. In contrast, very few changes in gene expression were observed in MLL-ENL expressing adult HPCs. Known targets of MLL-ENL, such as HoxA9, were more highly induced in neonatal HPCs than in adult HPCs, though expression was observed in older mice. Altogether, our data demonstrate a dynamic relationship between developmental stage and the transcriptional response to MLL-ENL, and they may help account for the high percentage of MLL rearrangements observed in infant leukemia. Furthermore, our findings have opened new lines of investigation into the relationship between the epigenetic landscapes of developing HSCs/HPCs and the programs that drive leukemogenesis.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal